Silver-based coating systems for electrical connector systems
A new coating system consisting of hard silver with antimony as an alloying element and graphite as a dispersion material has been developed for electrical connector systems in electromobility that are subject to high thermal and mechanical stress.
Due to the growing interest in electromobility and the increasing use of electrical contacts for sensors and electrical components, the need for reliable electrical plug contacts is increasing. These are subject to stresses caused by friction and temperature loads while at the same time requiring low contact resistance. Silver is the material with the best basic properties for this, and its application-relevant properties can be significantly improved by alloying or using dispersion materials. The new coating system, consisting of hard silver with antimony as an alloying element and graphite as a dispersion material, ensures long-term stability at temperatures of up to 200°C. It also has excellent friction coefficients. It also has excellent friction values and low contact resistance. These characteristics have been proven both on test benches and in test environments close to vehicles.
Increased requirements for silver-based electrical connector systems
The share of battery electric vehicles reached new record figures in 2023, driven in particular by record sales in China[1]. This is very likely to continue in 2024 and the following years. This rapidly advancing market penetration in large parts of the world and the associated rapid technical progress in the field of electromobility and automated driving are leading to sharply increasing demands on electronic components in vehicles. In the area of silver-based electrical connector systems, this manifests itself in increasing mechanical loads in the form of a high number of mating cycles and a shift in the temperature load spectra - individually or in combination with each other - with a simultaneous demand for low electrical contact resistance. The specific requirements for the silver layer can vary depending on the area of application: For example, applications for specific connectors in the engine compartment are also conceivable in combustion vehicles, which should be able to achieve up to 50 mating cycles at a currently targeted temperature load of approx. 180 °C. In the area of the charging socket, on the other hand, the requirement is for up to around 10,000 mating cycles at a maximum temperature load of 150 °C[2]. In addition, higher temperature loads are also expected here in the future.
Need for a diffusion barrier
For physical reasons, electroplated fine silver coatings alone are not able to fulfill this multitude of different conditions at the same time. Why is this the case? Fine silver has the distinct property of cold welding[3]. This can be a welcome property for some applications, such as press fits, but is completely undesirable for detachable plug connections with a high number of mating cycles. In addition, silver and copper show a very pronounced diffusion behavior into each other, so that a diffusion barrier is necessary for electrical contacts[3]. This is typically realized by a thin intermediate layer in the form of nickel. However, this creates a further problem: the delamination of the silver layer from the nickel diffusion barrier in the temperature range above 150 °C due to the formation of nickel oxide at the silver-nickel interface. This is due to the pronounced diffusion behavior of oxygen in silver at temperatures above 160 °C[3]. Due to the high demands placed on electrical connectors and the physical properties of silver, there is therefore a great need on the development side to be able to offer concrete, cost-efficient solutions for these problems described above, which rely on other precious metals such as palladium, for example, which drive up costs.
In this context, silver-graphite dispersion layers for sliding contacts in medium and high voltage have been developed for many decades and have been adapted and optimized over the years to meet new technical requirements [4-6]. Due to their lubricating properties, the graphite particles incorporated into the silver layer ensure a significant reduction in the friction coefficient of the layer and thus also prevent the ability of cold welding known from fine silver. In this way, the requirement for a high number of mating cycles can be achieved with low contact resistance and high electrical conductivity.
What remains unsolved with silver-graphite dispersion layers, however, is the fulfillment of the requirement for the layer system with regard to stability under high temperature loads. This is solved by introducing a diffusion barrier between the nickel and silver-graphite layer. This diffusion barrier consists of a functional antimony-containing hard silver layer. In principle, other alloying elements such as Se, In, Te, Bi or similar elements or combinations thereof are also suitable for this purpose in order to effectively reduce diffusion paths for oxygen in silver or to greatly impede them.
Results of the shift sequence
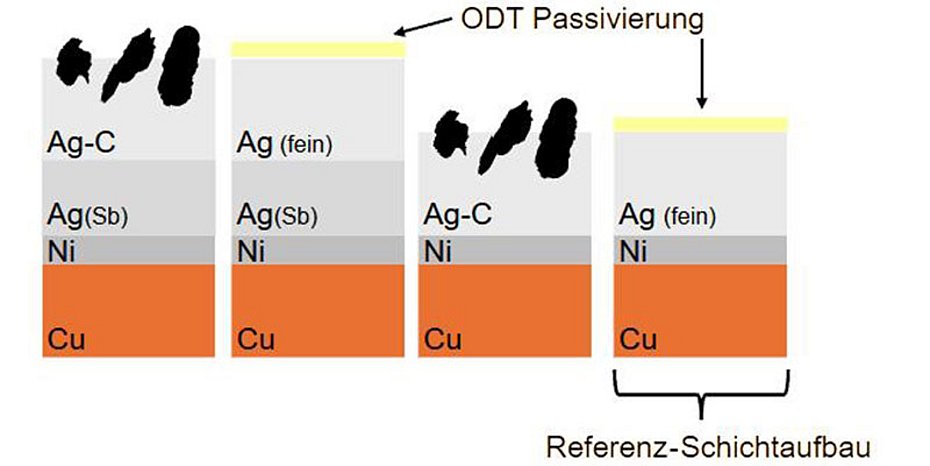
The results of such a coating sequence with an antimony-containing hard silver intermediate layer and silver graphite as the final layer (SLOTOCONNECT HT 4200 CF) or with ODT passivated fine silver as the final layer (SLOTOCONNECT HT 4200) and without an antimony-containing hard silver intermediate layer and silver graphite as the final layer (SLOTOSIL SG1910) are presented below. ODT-passivated fine silver is used as a series reference. For the corresponding tests to characterize the coating sequence, the corresponding coating sequences were electroplated onto nickel-plated copper test panels. The test specimens were coated with approx. 4 µm antimony-containing hard silver and approx. 4 µm silver-graphite or approx. 4 µm fine silver as a final layer or only silver-graphite with a layer thickness of approx. 8 µm. Nickel-plated copper sheets were used as a reference with approx. 8 µm fine silver as the final layer. The fine silver end layers were also ODT passivated. The selected layer thicknesses are exemplary and can also be selected higher or lower depending on the technical requirements. The sample parts and the layer sequences are shown in Fig. 1.
The coated components were tested on a physical test bench (WECO-X) developed in cooperation with iChemAnalytics. One of the special features of this test stand is that the contact resistance can also be measured in-situ during the cyclic measurement of the coefficient of friction, so that a cycle- or time-resolved direct correlation between the coefficient of friction and the electrical contact resistance of the respective surface under investigation is possible. Such measurements are possible on four test specimens simultaneously. The corresponding test rig is shown in Fig. 2.
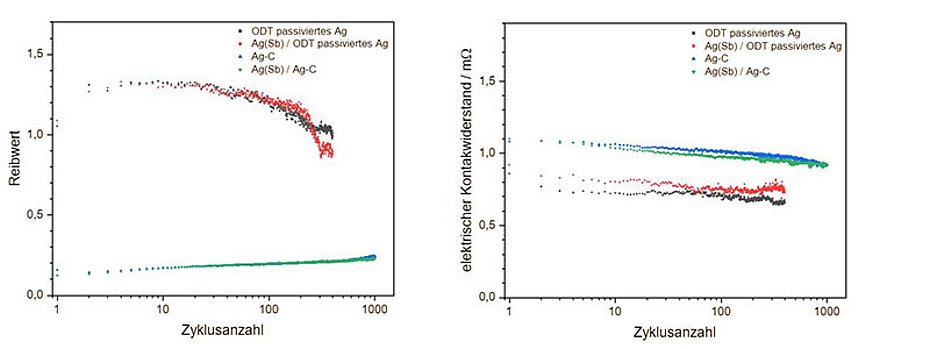
The coating systems shown in Fig. 1 a) on plate test specimens, see Fig. 1b), are used to assess the resistance to mating cycles. Silver spheres with a diameter of 3 mm were used as test specimens. The test specimens were tested at 23 °C for 1000 cycles each at a normal force of 1.5 N, a test frequency of 1 Hz, a deflection amplitude of 500 µm and a test current of 100 mA in the tribological test rig. The corresponding cycle-resolved measurement data of the coefficient of friction and the electrical contact resistance are shown in Fig. 3. Figure 3 a) shows that the initial coefficient of friction of the two test specimens coated with silver graphite as the final layer is initially between 0.1 and 0.2. As the number of test cycles progresses, the coefficient of friction increases linearly to around 0.26 after 1000 test cycles. The fine silver coating systems passivated with ODT initially show a significantly higher coefficient of friction of around 1.1, which rises to around 1.3 within the first few cycles, begins to fall after around 30 cycles and reaches around 0.9 (antimony-containing hard silver / fine silver coating system) or 1.1 (fine silver coating system) after 400 cycles, but is thus very clearly above the silver-graphite systems. The test specimens coated with silver-graphite show no evidence of wear after 1000 test cycles due to the lubrication by graphite. This can also be seen in the in-situ, cycle-resolved measurements of the contact resistance. The two test specimens coated with silver graphite as an end layer show that the initially measured contact resistance is around 1.1 mΩ and decreases linearly as the number of cycles progresses until it is around 0.9 mΩ after 1000 cycles - analogous to the coefficient of friction. This means that there is also no indication of significant wear with regard to the electrical contact resistance for the coating systems with silver-graphite as the final layer. The contact resistance behaves similarly for the silver reference system. The initial contact resistance drops from 0.9 mΩ to around 0.8 mΩ.
Testing the adhesive strength of the silver coating systems
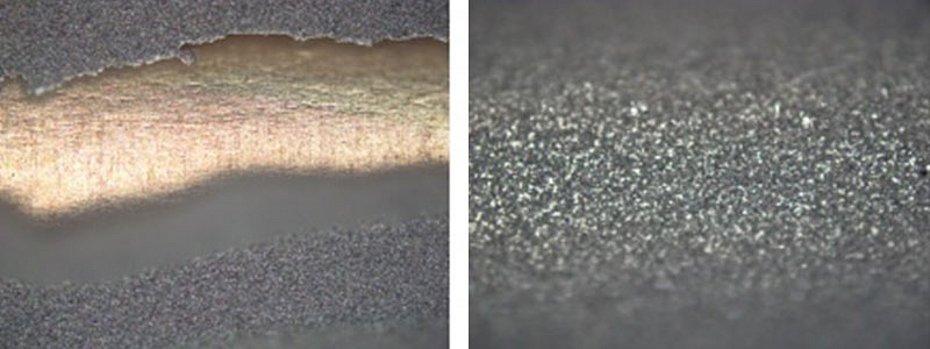
As described above, the stability of the coated components at high temperatures is of great importance for technical applications. This was investigated as part of temperature aging tests at 200 °C for 1000 h with intermediate withdrawals after 200 h, 400 h, 600 h and 800 h. For this purpose, the adhesive strength of the silver coating systems was evaluated by means of a classic bending test on the nickel-plated copper test specimens after temperature aging. It was found that the silver coating systems without an antimony-containing hard silver intermediate layer failed after 600 h due to local delamination of the nickel layer, see Fig. 4a). The test specimens with layer structures consisting of an antimony-containing hard silver intermediate layer and silver-graphite as the final layer or an antimony-containing hard silver intermediate layer and fine silver, on the other hand, passed the adhesion tests consistently, see Fig. 4b).
Shift system meets the technical requirements
The results presented here could also be independently reproduced by suppliers from the automotive sector. In summary, it can be concluded that the SLOTOCONNECT HT 4200 CF coating system fulfills the technical requirements with regard to the temperature stability, number of mating cycles and electrical contact resistance described above excellently and thus represents a cost-efficient solution without negatively influencing other required demands on a silver-based series connector system. If the properties of fine silver with regard to the maximum possible number of mating cycles can meet the requirements placed on the coating, the SLOTOCONNECT HT 4200 coating system is the optimum graphite-free solution. (OM-8/24)
Sources
[1] N. Carey, Global electric car sales rose 31% in 2023 - Rho Motion, Reuters, 2024.
[2] S. Berger, F. Talgner & R. Ziebart, Silber-Palladium Schichten als Kontaktoberflächen, Galvanotechnik,1 S31-S38, Leuze Verlag, 2021.
[3] H. Schmid & I. Buresch, Oberflächen für Steckverbinderkontakte, in Praxishandbuch Steckverbinder (Hrsg H. Endres), pp245-286 Vogel Communications Group, Würzburg, 2021.
[4] G. Clarsbach Behringer, H. Laub & S. Zjilstra, Cyanide, silver electrolyte and process for the electrodeposition of silver-graphite dispersion coatings and its application 1978.
[5] P. Rehbein & V. Haas, Contact surfaces for electrical contacts, EP1673836B1, 2010.
[6] A. Stadler, R. Sottor, R. Wagner, C. Diandl & S.Heitmüller, Silver electrolyte for the deposition of dispersion silver coatings and contact surfaces with dispersion silver coatings, EP3797184B1, 2023.
Contact
Dr.-Ing. Max Schlötter GmbH & Co. KG
Talgraben 30
73312 Geislingen an der Steige (Germany)
Phone: 07331 205-0
E-mail: info@schloetter.de
www.schloetter.de
About Schlötter
Dr.-Ing. Max Schlötter GmbH & Co. KG has more than 100 years of experience in the development of electrolytes for electroplating and the corresponding plant technology. As a specialist company for electroplating technology, they offer solutions from a single source for chemistry, plant technology and service. The aim is to enable innovative and high-performance surfaces and coatings and to support customers in the development of new coating systems.