UDE: Producing single-atom catalysts by modifying the surface
Oxygen and chlorine gas generation without precious metals: The production of single-atom catalysts has so far usually been associated with precious metals that are anchored to a solid surface. Researchers have now shown that such structures can also be formed electrochemically.
Single atom catalysts combine the advantages of homogeneous and heterogeneous catalysis. They are highly selective and can be easily separated from the reaction mixture. Until now, their production has generally been associated with precious metals that are anchored to a solid surface. Researchers led by the University of Duisburg-Essen have now shown that such structures can also be formed electrochemically - independently and without precious metals. Their findings, published in the journal JACS, open up new avenues for the simpler, more sustainable production of catalytically active materials. MXenes are a class of two-dimensional materials that were only discovered in 2011. Theoretical studies previously predicted that they are not catalytically active in anodic processes. Researchers led by Prof. Dr. Kai S. Exner, Head of Theoretical Catalysis and Electrochemistry at the University of Duisburg-Essen (UDE), have now been able to disprove this theory using multiscale modeling.
Changing the electrical potential of MXenes on their surface
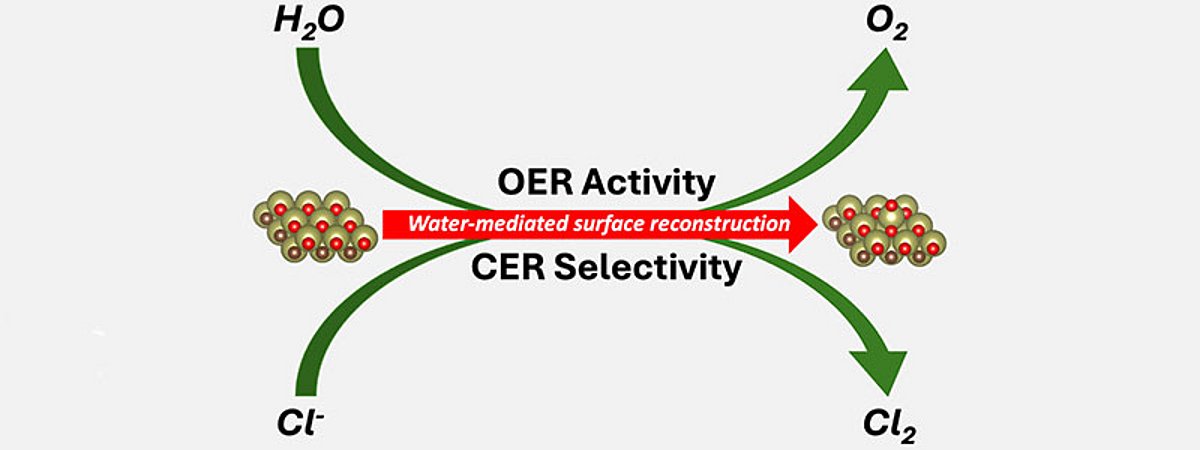
The scientists discovered that when an electrical potential is applied to MXenes, their surface changes into a brush-like structure: atoms of base metals migrate out and form so-called "SAC-like structures" (single atom catalysts-like). These mediate two important reactions: the development of oxygen and chlorine gas. The result is a material whose surface has catalytically active sites without the addition of precious metals. "We were able to conclude that MXenes behave similarly to enzymes in an electrochemical environment: Applying an electrical potential creates their active sites directly in the process," explains Exner.
Production of single-atom catalysts without precious metals
The team was also able to show that the resulting structures work selectively: If water and chloride ions are in the reaction environment at the same time, only chlorine gas evolution takes place. This is a central process in the chemical industry, which supplies over 70 million tons of chlorine gas (Cl2) worldwide every year. Cl2 is required for the production of medicines, plastics and batteries as well as for the treatment of water. If only water is available to the active MXene surface, however, it releases oxygen (O2) - an important step for the formation of green hydrogen in an electrolyzer. This discovery can significantly simplify the production of single-atom catalysts. The elimination of expensive precious metals also reduces costs and dependencies. Researchers from the University of Barcelona (Spain) and scientists from Ruhr Explores Solvation, or RESOLV for short, were also involved in the study. RESOLV is a Cluster of Excellence of the University Alliance Ruhr.
