KIT: New surface material repels water almost completely
Researchers at the Karlsruhe Institute of Technology (KIT) and the Indian Institute of Technology Guwahati (IITG) have developed a surface material that repels water almost completely. Possible applications include self-cleaning surfaces in cars or buildings.
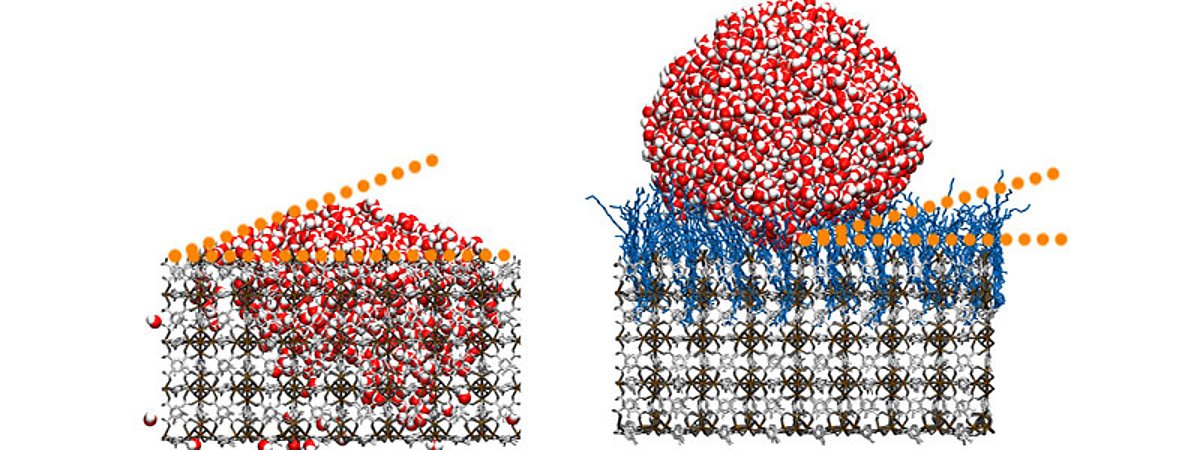
Using a completely new process, the researchers at KIT and IITG modified metal-organic frameworks (MOFs) - artificially designed materials with new properties - with the help of hydrocarbon chains. The resulting superhydrophobic, i.e. highly water-repellent, properties are of interest for use as self-cleaning surfaces that need to be robust against environmental influences, for example in cars or in architecture. MOFs (metal-organic frameworks) consist of metals that are linked by connecting struts of organic molecules to form networks with empty pores, similar to a sponge. Their volume properties - if you were to unfold two grams of this material, you would get the surface area of a soccer pitch - make them interesting for applications such as gas storage, carbon dioxide capture or new technologies in the field of medicine.
Superhydrophobic surfaces
However, the outer surfaces of these crystalline materials also offer unique possibilities, which the research team has now exploited with a new idea: they anchored hydrocarbon chains on thin MOF films. A water contact angle of more than 160 degrees was observed - the greater the angle that the surface of a drop of water forms with a substrate, the more water-repellent the material is. "Our method produces superhydrophobic surfaces with contact angles that are significantly higher than those of other smooth surfaces and coatings," says Professor Christof Wöll from the Institute of Functional Interfaces at KIT. "Although the wetting properties of MOF powder particles have been researched, the use of homogeneous MOF thin films for this purpose is a groundbreaking concept." The team attributes these results to the brush-like arrangement (polymer brushes) of the hydrocarbon chains on the MOFs. Once anchored to the MOF materials, these can form "tangles" particularly well - a state of disorder that scientists refer to as a "state of high entropy" and which is essential for the water-repellent properties. According to the researchers, this state has not been observed for anchored hydrocarbon chains on other materials.
Reduced adhesion enhances water-repellent properties
Remarkably, the water contact angle was also not increased by perfluorination of the hydrocarbon chains, i.e. by replacing the hydrogen atoms with fluorine. In materials such as Teflon, perfluorination leads to particularly water-repellent properties. In the case of the newly developed material, however, it has actually significantly reduced the water contact angle, according to the team. Further analyses in computer simulations had confirmed that the perfluorinated molecules - unlike the hydrocarbon chains - cannot assume the energetically favorable state of high entropy. In addition, the research team varied the surface roughness of their SAM@SURMOF systems in the nanometer range. This made it possible to further reduce adhesion. Water droplets then begin to roll off at extremely small angles of inclination, and the water-repellent or self-cleaning properties are significantly increased once again. "Our work also provides a comprehensive theoretical analysis linking unexpected experimental behaviors to the high entropy state of the molecules attached to MOF films," says Professor Uttam Manna from the Department of Chemistry at IITG. "This study will change the design and production of the next generation of materials with optimal hydrophobic properties." The study was published in the journal Materials Horizons(DOI: 10.1039/D4MH00899E).
